Pharmaceutical formulations are available as tablets, capsules, ointments, pills, oral liquids, aerosol sprays, etc. Even a single drug can be found in several different formulations which impart it different characteristics as per administration requirements .In case of solid formulations X-ray diffraction has been used as a non-destructive technique for testing of inconsistencies in manufactured batches, new drug development, and detection of impurities, degree of crystallinity and presence of polymorphism. In other words XRD serves as a fingerprinting technique for pharmaceutical formulations. It helps in optimization of manufacturing parameters, compatibility of excipients with active ingredients and controlling properties of dosage forms such as dissolutuion rate of active ingredients and stability behaviour under different environmental conditions.
The article discusses some common applications of the technique in drug manufacture and quality control .It is important to mention that XRD is a sensitive technique and errors in sample preparation can lead to wrong inferences. The sample collected should be representative of the material and with uniform distribution of particles. During sample collection and preparation the material should not be subjected to unwanted stress or other environmental conditions as such factors can result in adverse effect on the material characteristics.
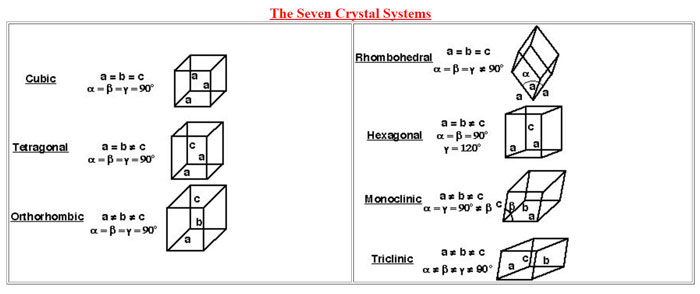
Identification
Conventionally the analysis is carried out by crushing the tablet or capsule gently in a mortar and pestle or a grinding mill. Vigorous grinding can introduce mechanical stress leading to phase transitions. For small sample quantities capillary mounts can be used. Today modern diffractometers have provision to correct for the curvature when examining tablets or capsules with curved surfaces. XRD analysis can help determine the uniform distribution of tablet ingredients. Such distribution can establish the preferential treatment of surface modifying agents such as pigments or lubricants which can influence shelf life of a formulation, its dissolution rate and disintegration behaviour. Accessories are also available to conduct studies under variable temperature and humidity.
Degree of hydration or solvation
Hydrated or solvated molecules have different characteristics. Thermal techniques such as TGA and DSC are being used to determine the number of molecules of hydration or solvation but XRD helps easy correlation of such states with crystal structure. Variable temperature accessories further provide an idea of temperature or humidity on associated properties of such materials.
Amorphous content
A drug substance can contain excipients which are amorphous in nature. In comparison with crystalline materials an amorphous material shows instability and transforms to crystalline phase over time due to environmental conditions during storage. Solubility of a drug increases with increasing amorphous content so it becomes necessary to quantify the amorphous content. X-ray diffraction offers a useful tool for quantifying the amorphous content. This is achieved by comparison of the intensity ratio between the peak of crystalline component with the hollow intensity of the amorphous content.
Polymorphic impurity profiling
A formulation can contain polymorphic impurities, products of degradation and excipients which can have a bearing on the functionality, stability and effectiveness of the active ingredient. Different polymorphs of the same compound exhibit different properties. Compression forces during tablet production can cause changes in composition of such compounds. X-ray diffraction is an established method for estimation of such substances
Pharmaceutical analysis poses quite a few challenges due to presence of a broad range of materials, their orientations and mixed crystallinity. However, XRD provides useful information and in combination with other contemporary techniques such as TGA, FT-IR, DSC, NMR,etc can provide useful details on pharmaceutical formulations.