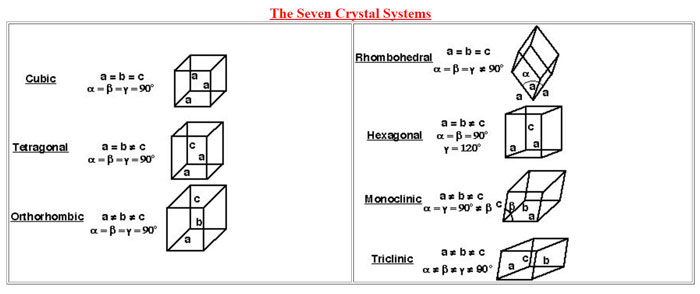
X-ray diffraction is a valuable tool in the hands of the analytical chemist for material characterization which is based on arrangement of atoms in the crystal lattices. The biggest advantage is that it is a non-destructive technique and the sample can be reused for further investigations by other means. Samples can range from symmetrically ordered atomic arrangements to random arrangements as in amorphous substances. The common applications of x-ray diffraction studies include crystal lattice dimensional analysis, grain size, crystal defects and residual strain.
X-rays interact with solid materials to generate diffraction patterns. Data on diffraction patterns resulting from interaction of x-rays with inorganic and organic solids shows definite patterns which can be used as fingerprints in identification of such materials. Diffraction data base is maintained by the International Centre of Diffraction data (ICDD) which was formerly known as Joint Committee on Powder Diffraction Standards (JCPDS). Such reference data can be purchased direct from ICDD or through
x-ray diffraction instrument suppliers.
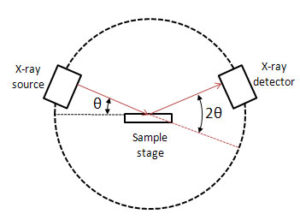
X-ray diffraction studies easily distinguish between single crystal orderly arrangements of atoms to polycrystalline arrangements. The atomic planes of crystal lattices responsible for scattering of x-rays are their reflective surfaces. Scattered beams when in phase interact constructively and intensities are maximum at particular angles. Such reflecting patterns on reaching the detector will generate a response which can be matched with the pattern from standard materials.
It is interesting to note that under the influence of heat small particles anneal to form larger aggregates. This becomes apparent as peak intensities get enlarged and help distinguish nano-particles from larger aggregates of particles.
Applications of XRD
X-ray diffraction is a powerful tool for characterization of
nano- materials, bulk materials and thin polymeric films.
Phase studies
Powder crystallographic studies help characterization on the basis of chemical composition of materials. Such studies provide valuable details on phase transformations resulting from subjecting materials to extremes of temperatures.
Degree of crystallization
Polymeric materials often exhibit mixed behaviour as they can be partially crystalline and partially amorphous. The degree of amorphous content can vary with the conditions used during their processing. The more the amorphous content the greater will be the peak broadening so the ratio between the peaks of pure crystalline standards and polymeric materials will give an idea on the degree of crystallinity in the polymeric sample.
Residual stress
Stress is defined as force acting on a material per unit area and any deformation per unit length is referred to as strain. Residual stress is the stress that remains in the material when the force responsible for it is removed. In synthetic materials residual stress results from material treatment processes such as machining, welding, heat quenching, etc. On the other hand in geological samples such stress could be the result from natural rock dynamics under the earth’s surface. X-ray diffraction is helpful in studies on residual stress introduced in materials through artificial or natural processes.
X-ray diffraction also helps study the dominant or preferred orientation of polycrystalline aggregates. Such information is beneficial in relating orientations of aggregates or texture to the desirable properties of materials.
In conclusion it can be said that x-ray diffraction studies provide valuable information which can help characterize both manufactured as well as naturally occurring materials.